Connecting academia and industry for innovative drug repurposing in rare diseases: it is worth a try
Abstract
There are different approaches to drug repurposing (DR) depending on the status of the repurposable drug/molecule (approved, investigational, withdrawn, shelved), the context, and the stakeholders involved. The purpose of this perspective paper is to highlight the complexity of academia-industry collaborations in DR for rare diseases and go beyond stereotypes to consider realistic and mutually reinforcing cooperation among various stakeholders, including not only academia and industry but also regulators, legal experts, and payers, leading to benefits for patients with unmet medical needs. Key questions are addressed through the presentation of select DR case studies. Some ongoing and promising European and international initiatives are introduced and some recommendations are proposed.
Keywords
INTRODUCTION
Drug repurposing (DR) can be defined as the process of identification of new uses for approved or investigational drugs that are outside the scope of the original medical indication. It is considered to have significant advantages over developing an entirely new drug: fewer risks, lower costs, and shorter timelines. Public-specific programs promote DR initiatives while pharmaceutical companies integrate them as a strategy in the life cycle management of their products. Indeed, DR represents a relevant opportunity to find treatments, especially in the field of rare diseases, where 95% of the 7,000 to 8,000 rare diseases have no approved treatment. A few well-known success stories are good illustrations of successful academia-industry DR collaborations, which may be viewed as the tip of the iceberg. There are also bitter and anonymous failures of collaboration.
The purpose of this perspective paper is to explore academia-industry collaborations in DR of medicinal products for rare diseases through their main challenges and successes and through select DR case studies. Key questions are raised to understand why, when, and how to implement a relevant collaboration. Some recommendations are also proposed.
DEFINITION OF DRUG REPURPOSING
DR is also known as drug repositioning, reprofiling, retasking, rediscovery, rescue, etc. While reflecting on different types of situations encountered and described in the literature[1], all these terms are synonyms and refer to a similar process that identifies new indications for approved, investigational, failed or shelved drugs/compounds. At present, the terms are all used interchangeably. This article will refer to all terminologies as “drug repurposing” for consistency purposes. As of November 1, 2022, a search on PubMed listed 1973 results mentioning “drug repurposing” and 861 results mentioning “drug repositioning” for 2022.
DRUG REPURPOSING PROVIDES AN OPPORTUNITY FOR RARE DISEASES
Much has been said about DR over the last two years with the COVID-19 pandemic. An editorial entitled “COVID-19 and the DR Tsunami”, published in July 2020, reflects an unprecedented wave to repurpose existing drugs[2]. The aim is to vastly accelerate the usually long approval process of drugs[3], which is a common goal and an objective also shared with rare disease patients desperately waiting for treatments.
Today there are an estimated 350 million people worldwide living with a rare disease. 7,000 to 8,000 rare diseases have been identified, with 250-280 new diseases discovered annually. Only about 5% of rare diseases are estimated to have approved treatments.
In Europe, between 2000 and 2021, 245 orphan drugs, so named for the rare diseases they treat, have been approved. They cover a total of 148 rare diseases[4]. About 20% of orphan drugs are repurposed drugs[5].
There is an urgent need for treatments[6] as most of the rare diseases are life-threatening. DR may be a particularly attractive option for the development of treatments for rare diseases. Of note, oncology and infection are together the most common disease areas for DR so far[7].
SELECT EXAMPLES OF SUCCESSFUL DRUG REPURPOSING
The most famous case of DR is probably the development of sildenafil which is also a good example of both investigational and approved DR[8]. Sildenafil, a phosphodiesterase-5 (PDE5) inhibitor initially explored as a treatment for angina pectoris and hypertension by Pfizer, was tested unsuccessfully in these diseases and eventually demonstrated efficacy in treating erectile dysfunction (with the trade name Viagra®) and then pulmonary arterial hypertension (with the trade name Revatio®)[9].
The clinical trials in angina, which had started in 1989, were disappointing, and sildenafil failed in the Phase II trial. However, a side effect (induction of penile erection) was serendipitously found during the Phase I and II clinical trials and redirected sildenafil to the treatment of erectile dysfunctions (ED). This indication was approved in Europe and the United States in 1998.
After its serendipitous repurposing for erectile dysfunctions, sildenafil was then repurposed (on purpose this time) for a rare disease called pulmonary arterial hypertension[10]. This indication was approved in Europe and the United States in 2005[11].
Of note, sildenafil acts on the same target: phosphodiesterase 5 (PDE5), to treat both erectile dysfunction and pulmonary arterial hypertension. PDE5 is a key enzyme involved in the regulation of cyclic guanosine monophosphate (cGMP)-specific signaling pathways in normal physiological processes, such as smooth muscle contraction and relaxation.
Sildenafil was originally tested for angina pectoris, a chest pain associated with coronary heart disease. Since PDE5 hydrolyzes cGMP in the cardiopulmonary vasculature, researchers aimed to establish a new anti-anginal agent using PDE5 inhibitors to prolong cGMP activity and promote vasodilation of the coronary arteries. However, early unconvincing results suggesting PDE5 was minimally present in cardiomyocytes led to the abandonment of this research approach[12]. PDE5 is the predominant PDE in the corpus cavernosum. The catalytic site of PDE5 degrades cGMP, the key second messenger in the mediation of penile erection. In men with erectile dysfunction, selective inhibition of PDE5 leads to an increase of cGMP in corpus cavernosal tissue and improves erectile function.
Persistent pulmonary arterial hypertension (PAH) in newborns has been shown to be linked to PDE5 overexpression and overactivation. PAH is characterized by increased pulmonary vascular resistance due to vasoconstriction of the small pulmonary arteries and arterioles. In blood vessels, cGMP relaxes vascular smooth muscles leading to vasodilation and increased blood flow. By inhibiting PDE5 and raising the intracellular cGMP, sildenafil is an effective pulmonary vasodilator.
Understanding the mechanisms of action (MOAs) of drugs is critical not only for drug development but also for DR.
The identification of drug MOAs has been primarily based on pharmacological experiments.
Identification of MOAs through biological pathways involving a drug and its targets is a more recent alternative approach to point to diseases not currently treated by a drug, to predict new uses of existing drugs and to repurpose them. Such an approach can be of real interest also for drugs with unknown underlying mechanisms.
In fact, biological pathway analysis based on drug targets (genes and proteins) may reveal new MOAs and also new clinical functions of existing drugs[13]. Alpelisib provides a good example of innovative DR in this respect by illustrating how the analysis of a biological pathway can lead to the treatment of diseases other than those initially investigated.
Alpelisib, an inhibitor of PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha), was primarily developed for the treatment of PIK3CA-mutated cancers and then repurposed for the treatment of PIK3CA-related overgrowth spectrum (PROS).
Cancer-associated PIK3CA gene mutations result in the production of an altered p110 alpha subunit. Strongly and permanently activated due to the presence of a gain-of-function mutation in PIK3CA, p110 alpha allows the Phosphatidylinositol 3-Kinase (PI3K) to signal without regulation, promoting oncogenesis and tissue overgrowth[14]. Patients with PIK3CA-mutated, hormone receptor (HR)-positive/HER2-negative tumors have poor outcomes and resistance to chemotherapy[15].
Developed by Novartis, alpelisib was approved by the United States Food and Drug Administration (FDA) as Piqray® in combination with fulvestrant for advanced or metastatic breast cancer with a PIK3CA mutation. Fulvestrant is a selective estrogen receptor antagonist which works both by down-regulating and degrading the estrogen receptor. Piqray® is used with fulvestrant after hormone treatment used alone has failed.
Activating PIK3CA mutations are also found in overgrowth syndromes, collectively known as PIK3CA-related overgrowth spectrum (PROS). PROS includes a group of genetic disorders and various clinical entities that leads to the overgrowth of various body parts due to mutations in the PIK3CA gene. The CLOVES (Congenital Lipomatous Overgrowth, Vascular malformations, Epidermal nevi, and Skeletal anomalies) syndrome, a rare disorder first described as a distinct syndrome, is, for instance, one subtype of this spectrum.
The severity of PROS is highly variable, ranging from localized overgrowth to severe, extensive, and life-threatening overgrowth affecting major vessels and/or critical organs.
PIK3CA-related cancers and PROS share the same pathogenetic mechanism and almost the same PIK3CA mutational profile[14].
PI3K/AKT/mTOR pathway is involved in many biological processes, including cell metabolism, proliferation, survival, and growth; deregulation of PI3K/AKT/mTOR pathway functioning, in the sense of overactivity, promotes oncogenesis and tissue overgrowth[16].
It is then relevant to investigate the potential of some anticancer drugs to treat rare overgrowth syndromes such as PROS.
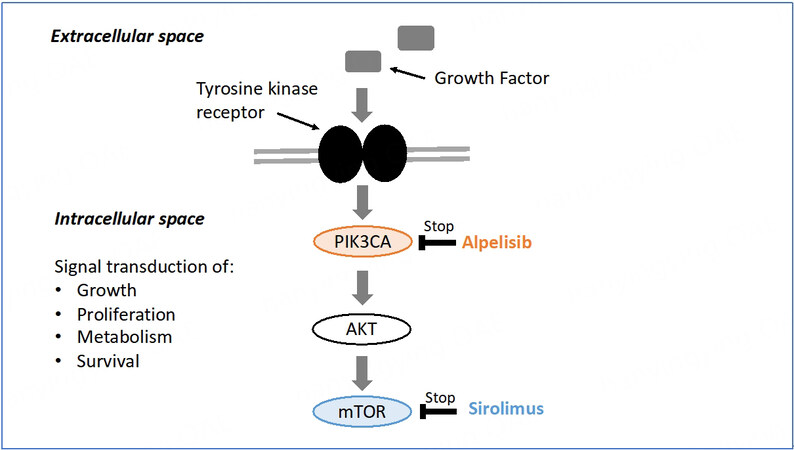
As in cancer, PIK3CA mutations in PROS occur as post-zygotic events, but unlike in cancer, these mutations arise during embryonic development[17]. Different components of the PI3K/AKT/mTOR signaling pathway can be specifically targeted to treat PROS. The PIK3CA protein is involved in the first intracellular signal transduction step of the mTOR pathway. Alpelisib acts at the top of the PI3K/AKT/mTOR signaling pathway and sirolimus, a direct inhibitor of mTOR, at the end of the PI3K/AKT pathway [Figure 1]. Both molecules were tested in PROS[14].
Figure 1. mTOR signal transduction pathway[15].
However, since activation of mTOR is responsible for some but not all of the biological effects caused by PI3K gain-of-function, alpelisib proved to be a more attractive drug candidate in PROS by providing a direct outcome on affected tissues while reducing the risk of off-target effects.
Alpelisib (BYL719) was in a phase III randomized double-blind, placebo-controlled study (SOLAR-1) conducted in patients with PIK3CA-mutated, HR-positive, human epidermal growth factor receptor 2-negative advanced breast cancer with a tolerable safety profile when Canaud’s research group decided to explore its therapeutic potential in PROS. After achieving impressive outcomes first on PROS mouse models and then on two patients suffering from extremely severe and life-threatening clinical manifestations of PROS/CLOVES syndrome, Canaud’s group was authorized to administer BYL719 to 17 additional patients with PROS. The study was published in 2018, supporting PIK3CA inhibition as a promising therapeutic strategy in patients with PROS[18].
Four years later, in 2022, the Food and Drug Administration (FDA) granted accelerated approval to alpelisib under the brand name Vijoice® to treat severe manifestations of PROS in patients aged 2 years or older. This indication for Vijoice® is approved based on real-world response rate and duration of response findings in 57 patients in the single-arm EPIK-P1 study[19], a retrospective chart review study. Real-world data (RWD) and Real-world evidence (RWE) may be supportive for regulatory decision-making in rare and ultra-rare diseases. Medical records with chart review may be useful tools to generate RWE. They also have limitations, and continued approval will be, however, contingent upon additional placebo-controlled studies that will confirm the clinical benefit of alpelisib in PROS patients.
ACADEMIA-INDUSTRY COLLABORATION: WHY, WHEN, AND HOW
The two successful DR examples mentioned above, alpelisib in PROS but also sildenafil in ED, are good illustrations of a successful academia-industry DR collaboration. In one case, investigators reported the side effects of sildenafil that became a repurposed indication in ED, and Pfizer drove the repurposing efforts; in the other case, an academic research team attempted independent repurposing of alpelisib in PROS, a new and rare indication further developed by Novartis.
If there are remarkable DR success stories that hit the headlines, there are also bitter and anonymous failures of collaboration.
Various reasons may explain the poor cooperation between industry and academia. These reasons need to be properly understood if we want to orient efforts in appropriate directions. Key questions may be addressed: why, when, and how to implement a relevant collaboration. They deserve clear answers through a broader and more structured debate involving all stakeholders. In the meantime, some suggestions can be made, and some existing initiatives at European (for some of them) and international levels can be reported to encourage and stimulate new opportunities.
WHY
Why get academia and industry to work together? There are many reasons to support such collaborations. Even if they have different expectations and constraints, academia and industry may be complementary and bring added value to each other in a highly regulated environment.
Academia as a source of innovation
Pharma’s business model fundamentally depends on product innovation to create value. Patent expiry and generic competition approximately every 10 years are real challenges for pharmaceutical companies which look to academia for valuable knowledge and innovation[20]. The contribution of academia is crucial in terms of basic research, identification and validation of new targets, and also in terms of clinical trials to evaluate the efficacy and safety of drug candidates. Academia and industry may be caricatured as the alpha and the omega as they have complementary enterprises throughout the drug value chain, from innovation to validation. Research for rare diseases starts in academia[21], development is shared between stakeholders, and commercial launch is managed by the industry.
Different expectations and constraints but working towards the same goal of improving the health of patients
It has become commonplace to highlight the gap between academia and industry in terms of cultures and practices. Although patenting is key for them both, the value of secrecy for pharma continues long after patent filing and grant. Pharmaceutical companies still consider results collected from clinical trials to be confidential information or trade secrets, even after submission to regulatory agencies. Academics, for their part, are under pressure to increase their publication count. The “publish or perish” culture is a well-known practice existing within academic institutions. But once the patent has been filed, the invention may be published. It is then crucial for a researcher to present his/her work at conferences, exchange with peers, and win grants.
Highly regulated drug development process
Academic researchers have neither the capacity nor the finance to develop a drug candidate until the launch of the finished product. Moreover, they are often unaware of what evidence needs to be submitted as part of an application for marketing approval or which complex drug development challenges will have to be addressed. Pharmaceutical companies operate in a very highly regulated area, from key nonclinical studies, Phase I-III clinical trials, chemistry, manufacturing and controls (CMC), and formulation activities to regulatory submissions, commercial launch activities, and life-cycle management[7]. Drug development requires a comprehensive and well-thought-out development strategy with a detailed roadmap.
Drug development is a risky and costly process
The time required for a new drug to be developed is about 10-15 years and the cost is around $ 2 billion. Despite such significant investments in time and money, approval rates for a new drug are about 10%. DR has been proposed as an interesting strategy with fewer risks, lower costs, and shorter timelines. Indeed, repurposed drugs are generally approved sooner (3-12 years), at reduced (50%-60%) cost and lower risk: approval rates for repurposed drugs are close to 30%[22]. However, in some cases, DR may be an expensive, time-consuming, and risky process[23]. Furthermore, it does not always succeed. Like de novo drug development, DR may also fail in late-stage development. That was the case for latrepirdine, originally developed and marketed as an H1-antihistamine for the treatment of skin allergy and allergic rhinitis. Latrepirdine was repurposed as a treatment for Huntington’s disease (HD). Following encouraging phase II trials and preliminary reports showing its neuroprotective functions and ability to enhance cognition in animal models, it failed to show efficacy in phase III trials in HD patients[24].
Some authors have underlined the importance of confirmatory validation studies for a successful translation of academic results to the industry before larger investments are made[25].
Such gaps between academia and industry should favor rather than discourage a move towards stronger collaboration. Both have their respective roles to play in a drug development process; there is complementarity and not duplication, and there may be mutual support learning from one another. Moreover, while repurposing previously relied on some serendipity, it has more recently evolved to continuous advances made in the field of data sciences and to approaches integrating -among others- computational assistance using Big Data and Artificial Intelligence[26].
WHEN
There is no ideal timing to start a collaboration
There are rather special circumstances leading to potential opportunities or not. For instance, one of the nightmares of a pharmaceutical company is to get safety issues during pivotal studies close to the approval stage. That was the case for alpelisib when Canaud’s team asked for the molecule to be tested in PROS. Although based on an exhaustive literature review and spectacular first results in mice and patients, the alpelisib request was badly timed. The phase III trial of alpelisib in breast cancer was still ongoing. The detection of a potentially serious adverse event during a clinical trial in PROS could have jeopardized the drug development for both indications.
Academia-industry collaboration may start as early as possible within a co-development framework or should be at least anticipated to avoid unexpected issues such as the unpleasant surprise for the industry to learn one of its drugs is unexpectedly repurposed by an academic team in an out-of-scope indication, or the huge frustration for the academic team who will not be able to develop a promising candidate owned by industry and not part of its strategy. It must be noted here that large pharmaceutical companies do not have the same expectations as smaller ones. They prefer late-stage development projects (Phase II and onwards) in which they have experience and which are also a way to mitigate the risks.
Line extension or label expansion is not the right time for a collaboration
DR may be part of an anticipated strategy from the industry in the early drug development process. Rather than DR, it is considered as line extension or label expansion that is conceived from the outset of the program. It is part of life cycle management (LCM) activities whose objective is to maximize the value of the products with new indications, improved formulation, new packaging, etc., and to allow for patent extension[27]. Only the marketing authorization holder of a drug can currently apply for an extension of its marketing authorization. When drugs are repurposed on the basis of on-target effects (as is the case with sildenafil and alpelisib), it means that work may be undertaken by the pharmaceutical company owning the molecule. Company data are not available to academic investigators.
Intellectual property considerations are a critical element when starting a collaboration
Drugs are protected by patents and supplementary protection certificates and may benefit from another form of market exclusivity, as in the case of orphan drugs (7 years in the United States, 10 years in Europe).
Patents can cover the existing product, its underlying chemical or biological make-up, as well as new indications, dosage regimes, or mechanisms of delivery. Supplementary protection certificates are intellectual property rights that serve as an extension to a patent right. They all give the owner the right to prevent others from making, using, or selling the drug without permission. Patent protection is critical for developing a new therapy.
It is challenging to obtain strong patent protection when repurposing compounds. By definition, the structure of a repurposed drug is known and a novel patent claim to the active pharmaceutical ingredient is not possible. Composition of matter (COM) claims, which exclude any use of the COM, are one of the classic and powerful claim types. They cannot be proposed to be repurposed drugs in the vast majority of cases. Repurposed drugs are eligible for patent protection through use claims or method of treatment (MOT) claims. MOT patents may be perceived as second-tier to composition patents; however, it seems to be, in some cases, a good way of protection and commercial success for some companies[28].
Intellectual property (IP) position is critical in ensuring the ability to achieve a collaboration based on partnership and dialogue. IP may fall into two major categories, leading to different issues: it can be active or expired.
IP is still in force
Alpelisib is an example of a successful academia-industry DR collaboration in this category leading to a treatment for patients, although the company was not interested in its development at the very beginning. Alpelisib was being evaluated for breast cancer at that time.
If an academic researcher has discovered a new indication for an approved or investigational drug, then early engagement with the potential industry partner is highly recommended since the company is the patent owner. It will be a crucial step in the process. The objective is twofold: to know if the company is interested in the development and to obtain access to the drug/compound and potential funding of studies performed under a material transfer agreement (MTA).
Pharmaceutical companies frequently engage in preclinical research collaboration with academics. Complex knowledge transfer processes and substantial challenges in terms of IP may arise. Generating new IP has to be discussed, anticipated, and validated in a consortium agreement in order to organize the rights and obligations of all stakeholders and protect the researchers’ discovery and IP[7]. If the company holding the IP agrees to provide the drug but refuses to provide funding to support preclinical or clinical programs, it will be difficult for the researcher alone to move forward with the drug, except if new patent protection can be obtained. New formulations or drug forms for a known active pharmaceutical ingredient (API) may be developed for a new indication and provide academics with an opportunity for patent protection. Academic investigators may also decide to perform clinical studies without the support of the company and with no guarantee -even if the trial is successful- of a newly approved indication. Repurposing an approved API for a new indication would only benefit the original manufacturer who owns the IP for the composition. Scientific and nonscientific understandable reasons may explain the company’s refusal, such as insufficient remaining patent life, a lack of expertise and interest in the new therapeutic area, or anticipated regulatory risks. A recent study explored innovative mechanisms to fund independent clinical research initiated and led by researchers from academia with repurposed medicines[29]. Focus is put on social impact bonds, crowdfunding, or public-private partnerships to conduct expensive phase III clinical trials with, again, no guarantee of successful achievement.
IP has expired
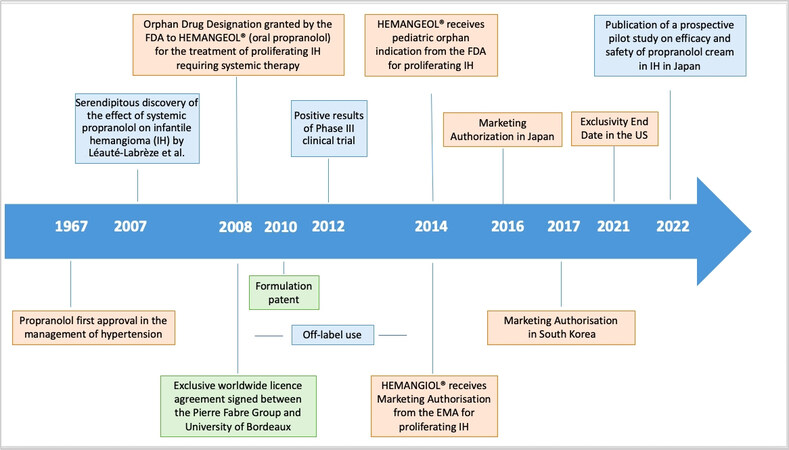
In this case, the drug is off-patent or generic and widely available at a low price. Propranolol is an example of a successful academia-industry DR collaboration in this category leading to a new treatment for patients [Figure 2].
Propranolol, a non-selective beta-blocker patented in 1962, was granted FDA approval in 1967 as an antihypertensive drug. When the Léauté-Labrèze team from the Bordeaux University Hospital showed its antiangiogenic therapeutic properties in 11 cases of infantile hemangioma (IH) in 2008, it was available as a generic[30]. 4.5% of birth have infantile hemangioma. Most infantile hemangiomas resolve spontaneously, but 12% are complex and severe forms[31] with functional and even life-threatening complications, such as breathing difficulties and requiring systemic treatment. Following this serendipitous discovery by the Léauté-Labrèze team, Pierre Fabre Dermatologie Laboratories and the University of Bordeaux formed a partnership in 2008 to co-develop and launch Hemangiol® in Europe and Hemangeol® (a different brand name for the same oral formulation) in the United States in 2014 for the treatment of proliferating infantile hemangioma requiring systemic therapy. For information, the brand name Hemangiol® was changed into Hemangeol® in the United States in accordance with FDA’s best practices in developing proprietary names guidance, according to which “io” is used as an infix to suggest a high iodine content[32]. In terms of organization and assignment of tasks, resources, and operational skills, the company took charge of the regulatory dossier, including the development of a pediatric formulation and a pivotal study performed on 460 babies between five weeks and five months of age at treatment initiation. The objective of the study was to select the right dosage and confirm the efficacy and tolerance of propranolol in infants with proliferating IH requiring systemic therapy. The clinicians of Bordeaux University Hospital were involved in the study design and management as well as the drafting and publication of the manuscript[33].
Pharmaceutical companies may be reluctant to initiate collaborations with academia to repurpose off-patent drugs. Once drugs lose patent protection, generics can be allowed for sale, leading to off-label use and commercial risks. As it was possible to dissolve commercially available propranolol tablets or to use a non-approved preparation of liquid propranolol made up by the hospital pharmacy, off-label use was a big challenge[34] with risks of inappropriate dose and use of excipients non-recommended for infants, since the therapy was adapted from adult dosage to pediatric populations without an appropriate formulation for young children. However, different actions may be put in place to protect companies.
Patents are the first step. For propranolol in IH, new patents were granted to academia (second medical use patent in 2008) and industry partners (formulation patent in 2010), offering protection for 20 years until 2028 and 2030, respectively.
In addition to patent protection, regulatory agencies are offering incentives for the development of orphan and pediatric drugs for rare diseases, such as Pediatric-Use Marketing Authorization (PUMA) in Europe and Orphan Drug Designation (ODD) in the United States.
Thanks to PUMA, drugs can benefit from ten years of market protection, including 8 years of data exclusivity plus an additional two years of market exclusivity[35].
An Orphan Drug Designation is a status delivered by the FDA in the United States or the EMA in Europe to medicines developed for rare diseases; it may be requested by sponsors for a previously unapproved drug or for a new orphan indication for an already marketed drug. It provides incentives including tax credits for clinical trials, exemption from user fees, and market exclusivity after approval of the drug. In 2008, an ODD was granted by the FDA to Hemangeol®, which could benefit from a seven-year market exclusivity starting in 2014 when the drug was approved. ODD was not granted in the European Union for Hemangiol® because of “salami slicing” which denies the possibility of restricting the target population to severe forms only (100% of IH are taken into account in the European Union vs. only 12% of them in the United States).
Other Marketing Authorizations (MA) have also been granted to the drug in other countries, such as Japan in 2016 and South Korea and Australia in 2017. However, MA does not mean market access and patient access. Each country has individual requirements for pricing/reimbursement with independent Health Technology Assessments (HTAs). If the submitted data package does not support a positive decision from one country’s HTA, then the product is at risk of partial reimbursement or even no reimbursement in that country.
A cost-utility analysis was conducted in 2015 in Italy to assess the costs and clinical benefits of Hemangiol® 3.75 mg/mL, an oral solution in comparison to corticosteroids considered as the standard of care in the treatment of proliferating infantile hemangiomas. Propranolol was considered cost-effective compared to corticosteroids from the Italian National Health Service perspective[36].
Propranolol is a good use case outlining the success and failure scenarios of DR for all the reasons outlined above. DR relies on a real strategy involving actors dealing with different constraints but aiming at the same final expectation.
Many lessons can be learned from it, although not always possible to be replicated. An effective IP strategy is an essential tool in identifying risks and opportunities when repurposing medicines and setting out on new projects[37], but it is only part of the puzzle. Market access strategy is critical to therapy launch success. Landscape assessment is still more relevant in the rare disease ecosystem where literature and natural history are limited. Close collaboration with clinicians is essential to better understand the burden of the disease and patients’ needs and work together on the clinical development program to optimize pediatric formulation and patient outcomes keeping in mind the expectations of regulatory agencies and payers.
A paper published in 2022 compares the price of Hemangiol® approved in IH and the price of its off-label alternative, not approved in the indication, in Germany[38]. It underlines the clinical development and registration program value taken into account by payers in the price obtained by the approved product. The price difference is significant but relatively lower for Hemangiol® compared with two other orphan drugs presented in the paper.
Although the results may not be generalized, they may foreshadow a positive return on investment for Hemangiol®. In any case, it is a success story with an approved treatment covering an unmet medical need for babies and infants with severe IH.
HOW
Implementing a relevant academia-industry collaboration requires a strategy to move forward. There are many opportunities for industry to partner with academia and vice versa. There is a wide range of forms varying in the extent of involvement and risk taken by the stakeholders. Identifying research opportunities, applying to private-public calls for proposals, forming an early-stage partnership, developing new methods for the design of small population clinical trials, understanding the natural history of the disease to better treat it, or advancing real-world evidence to support payer decisions are just a few examples of it. How to do it is the key question. A case-by-case answer is probably the best answer since there are so many different factors to consider for complete and successful achievement. Such factors are related to the stakeholders themselves, their profile, skills, interest, their willingness, and commitment, but they are also dependent on the candidate repurposing drug, its IP and freedom to operate, its safety and toxicity profile, and the data already accumulated toward gaining regulatory approval[27].
A brief overview of key suggestions and current initiatives in DR is given hereafter.
Educational program for academia
Academic researchers need to be guided and trained in IP, regulatory procedures, or market access to be able to understand/challenge the constraints and expectations of the industry. Technology transfer offices (TTOs) or neutral and trusted third parties (public or private) may support them to this end. First of all, it is extremely important for academia to understand industry needs when dealing with practical problems to which they can apply their knowledge. Academic research may be closely linked with practical implications in an industrial environment[39]. It is also important for academia to be aware of the pharmaceutical development of a drug and for the industry to be sensitized to the key role and importance of publications, grant approvals, and international networking for academia, notably for Ph.D. students and young researchers. There is a real need for mutual knowledge of expectations and constraints from both academia and industry in order to facilitate interactions and enable fruitful collaboration. Both have to learn from each other.
Pilot project from EMA and STAMP expert group
Another way of implementing relevant academia-industry collaborations is provided by some European initiatives dealing with DR. In October 2021, the European Medicines Agency (EMA) and the Heads of Medicines Agencies (HMA) launched a pilot project to support the repurposing of drugs. That project is a follow-up to the European Commission’s Expert Group on Safe and Timely Access to Medicines for Patients (STAMP) discussions. The objective is to support not-for-profit organizations, including academia, to generate sufficient evidence on the use of a well-established off-patent drug in a new indication. Academic researchers will be connected with regulatory agencies for early scientific advice and with companies for regulatory application. These investments must also be recognized in pricing and reimbursement policies to make access a reality for all patients[40,41]. Candidate projects were selected in June 2022.
Horizon Europe funding program for research and innovation
Being part of consortia involving teams from industry, academia, SMEs and patient associations may help academic partners build lasting collaborations and networks. A call for proposal “Tackling diseases (2021) (HORIZON-HLTH-2021-DISEASE-04)” was proposed under the Horizon Europe program and closed on September 21, 2021. 13 proposals among 253 submitted were related to the topic “Building a European innovation platform for the repurposing of medicinal products”. Among key outcomes, proposals had to address a widened collaboration to set up a sustainable platform and an innovative repurposing model integrating different components such as IP, methodological, scientific, regulatory, and financial aspects. Particular attention was given to supporting academic-driven research[42].
IRDiRC Task Force on drug repurposing
As a brief overview, one could also mention the recent IRDiRC Task Force on DR based on the IRDiRC Orphan Drug Development Guidebook[43]. The DR Guidebook is aimed at facilitating DR for rare diseases by identifying relevant available tools and practices, defining a list of Building Blocks (tools, incentives, resources, and initiatives), and creating detailed factsheets for each Building Block. The factsheets will be based on a systematic literature review and the expertise of the Task Force members. They will also provide advice and recommendations. A web application and an article are expected by the end of 2023.
DR platforms are an example of tools and resources to be found in this DR Guidebook. The DR platform established by the FDA is still available. It can be located now at cure.ncats.io. CURE ID (Infectious Disease) is a collaboration between the FDA and the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH). FDA and NCATS have made critical updates to CURE ID to be a more effective tool in the COVID-19 public health emergency.
Recommendations from a recent pharmacology and translational research round table
A recent “Pharmacology and translational research” round-table of the Giens Workshops[44] formulated and published a series of recommendations to help overcome sticking points and obstacles to DR[44]. The Giens Workshops are held in Paris every year; they are a think tank whose objective is to advance thinking on current issues in pharmacology and clinical research for therapeutic innovation and health technology assessment. The meetings are organized into round tables, each covering pre-defined themes (translational research; clinical research; health technologies including diagnostics, drugs, and medical devices; health and economics; organizational and regulatory aspects, and topical issues). Each round table aims to bring together experts from various backgrounds. In 2022 one of the round tables was dedicated to DR around the following key points:
Optimizing access to and use of databases
Databases are a basis for DR methods and have, therefore, to be designed in a proper manner to achieve relevant results. They can be used to predict drug-target interactions and discover new treatment benefits of the existing drugs. Access has to be facilitated to pharmacological databases (chemical and molecule libraries, associated pharmacological data). Although there are open-access databases that can be used for searching chemical, biological, and pharmacological data, it is not always easy to search into such specific databases. Another challenge is to have access to shelved industry compounds and their trial data when companies are reluctant to. It is then critical to simplify the procedures for accessing public databases and promote data sharing between public and private structures.
Improving awareness of intellectual property issues
DR can be done on shelved, failed compounds, live patents, and drugs sold on the market as well as generic drugs. DR always relies on a previously known drug, but each situation has its own challenges when considering collaborative research and development between pharmaceutical companies and academic researchers. If the repurposing project comes from academic bodies, they will have to discuss and negotiate with the pharmaceutical companies holders of the molecule involved in the project to convince them to agree on the methods for developing and sharing intellectual property.
It is then important to provide all researchers with better training in legal aspects of the drug development process. A proper DR project needs expert legal counsel to advise on the issues to identify risks and opportunities when setting out on new projects.
Anticipating preclinical and clinical requirements
The real feasibility of the project, the financial and human resources to be deployed, and the time needed to complete the project should always be analyzed and anticipated. There is a real need for tools or structures capable of providing advice on the model of the Scientific Advice/protocol Assistance of the EMA.
Improving the regulatory procedures for market access
Market Authorization is a regulatory process at the European level and does not necessarily mean market access in all Member States of the European Union. Some efforts should be made to get better alignment and synergies between regulatory and payers’ requirements. Regulatory agencies assess new treatments’ benefits and harms to the exclusion of economic considerations, whereas payers focus on effectiveness and economic consequences. In France, for instance, the doctrine of the High Authority for Health (HAS) Transparency Commission (TC) needs to evolve both regarding the methodology of potentially admissible trials and the more specific part of the so-called “relevant” clinical comparator. The HAS Transparency Commission’s main task is to assess medicinal products in order to provide recommendations on reimbursement decisions made by public authorities. Access to reimbursement in France follows Marketing Authorization and requires pharmaceutical companies to submit an application file to the HAS Transparency Committee (TC)
Creating a public-private partnership support structure on a European scale
The main recommendation made during the round table was the creation of a support structure capable of managing the most complex aspects of repurposing projects. A trusted third party is useful for initiating collaborations and providing the infrastructure and resources for academia and industry to work together. Such a support structure would have dedicated financial resources and expertise at all stages of development until the Marketing Authorization. It could even become the MA holder for the repurposed drug if need be. The Innovative Health Initiative (IHI) is a public-private partnership between the European Union and the European life science industries whose main objectives are to translate research and innovation into tangible benefits for patients. Such a structure could be used as a model applied to DR[45].
CONCLUSION
DR represents a real opportunity and a cheaper and faster way to find and develop new treatments for rare diseases. But DR is also associated with misconceptions and specific barriers (IP, regulatory, market access, and lack of incentives). It makes no sense to stereotype public-private partnerships and set one community against the other. Without research, there will not be any innovation; without regulatory and commercial development, there will not be any drug on the market. Although being far away from each other in terms of culture, constraints, and objectives, academia and industry can join forces and build off each other’s strengths to advance research and development of repurposed drugs for patients with rare diseases. This approach has already proved successful in several instances, but its potential is far from being fully exploited. Some fruitful collaborations have resulted in success stories that are widely publicized, but they do not fully reflect reality. The success rates of academia-industry collaboration vary depending on various factors (IP rights of the drug candidate, business strategy, Proof of Concept robustness, regulatory challenges, commercial aspects, and type of pharmaceutical company). Academic drug (re)discovery is a vital component, but the lack of knowledge of industry standards can make academics uncomfortable. Educational programs and guidelines could help them better understand industrial constraints and anticipate and adjust accordingly. There is also a need for new and sustainable DR business models involving not only academia and industry but also patient associations, regulators and payers to equitably address these questions. Some current European and international initiatives such as the pilot project from EMA and STAMP expert group, the call for proposal “Building a European innovation platform for the repurposing of medicinal products” within Horizon Europe or the IRDiRC Task Force on DR guidebook should encourage and stimulate new opportunities.
DECLARATIONS
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Murteira S, Ghezaiel Z, Karray S, Lamure M. Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: reassessment of nomenclature. J Mark Access Health Policy 2013;1:21131.
3. Galindez G, Matschinske J, Rose TD, et al. Lessons from the COVID-19 pandemic for advancing computational drug repurposing strategies. Nat Comput Sci 2021;1:33-41.
4. European Medicines Agency. Orphan medicinal product designation 2000-2020. Available from: https://www.ema.europa.eu/en/documents/other/orphan-medicines-figures-2000-2020_en.pdf [Last accessed on 7 Apr 2023].
5. European Medicines Agency. Support for development of orphan medicines. Available from: https://www.ema.europa.eu/en/documents/report/report-workshop-support-orphan-medicines-development_en.pdf [Last accessed on 7 Apr 2023].
6. Southall NT, Natarajan M, Lau LPL, et al. IRDiRC Data Mining and Repurposing Task Force. The use or generation of biomedical data and existing medicines to discover and establish new treatments for patients with rare diseases - recommendations of the IRDiRC Data Mining and Repurposing Task Force. Orphanet J Rare Dis 2019;14:225.
7. Begley CG, Ashton M, Baell J, et al. Drug repurposing: misconceptions, challenges, and opportunities for academic researchers. Sci Transl Med 2021;13:eabd5524.
8. Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci 2014;10:654-63.
9. Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 2006;5:689-702.
10. Jourdan JP, Bureau R, Rochais C, Dallemagne P. Drug repositioning: a brief overview. J Pharm Pharmacol 2020;72:1145-51.
11. Barnett CF, Machado RF. Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag 2006;2:411-22.
12. Roy S, Kloner RA, Salloum FN, Jovin IS. Cardiac effects of phosphodiesterase-5 inhibitors: efficacy and safety. Cardiovasc Drugs Ther 2021;Online ahead of print.
13. Pan Y, Cheng T, Wang Y, Bryant SH. Pathway analysis for drug repositioning based on public database mining. J Chem Inf Model 2014;54:407-18.
14. Pagliazzi A, Oranges T, Traficante G, et al.
15. Scherman D, Fetro C. Drug repositioning for rare diseases: knowledge-based success stories. Therapie 2020;75:161-7.
16. Chang DY, Ma WL, Lu YS. Role of Alpelisib in the treatment of
17. Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-associated
18. Venot Q, Blanc T, Rabia SH, et al. Targeted therapy in patients with
19. Canaud G, López Gutiérrez J, Irvine A, et al. LBA23 EPIK-P1: retrospective chart review study of patients (pts) with
20. Rosenblatt M. How academia and the pharmaceutical industry can work together: the president’s lecture, annual meeting of the American Thoracic Society, San Francisco, California. Ann Am Thorac Soc 2013;10:31-8.
21. den Berg S, de Visser S, Leufkens HGM, Hollak CEM. Drug repurposing for rare diseases: a role for academia. Front Pharmacol 2021;12:746987.
22. Hernandez JJ, Pryszlak M, Smith L, et al. Giving drugs a second chance: overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front Oncol 2017;7:273.
23. Fetro C, Scherman D. Drug repurposing in rare diseases: myths and reality. Therapie 2020;75:157-60.
24. Bharadwaj PR, Bates KA, Porter T, et al. Latrepirdine: molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Transl Psychiatry 2013;3:e332.
25. Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 2011;10:712.
26. WHO-EURO. Repurposing of medicines - the underrated champion of sustainable innovation: policy brief. Available from: https://apps.who.int/iris/handle/10665/342567 [Last accessed on 7 Apr 2023].
27. Cha Y, Erez T, Reynolds IJ, et al. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol 2018;175:168-80.
28. Conour J. ; 0. Why method of treatment patents for repurposed drugs are worth the investment. Available from: https://www.jdsupra.com/legalnews/why-method-of-treatment-patents-for-92813/ [Last accessed on 7 Apr 2023].
29. Verbaanderd C, Rooman I, Huys I. Exploring new uses for existing drugs: innovative mechanisms to fund independent clinical research. Trials 2021;22:322.
30. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51.
31. Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013;131:128-40.
32. U.S. Department of Health and Human Services; Food and Drug Administration. Best practices in developing proprietary names for human prescription drug products guidance for industry. Available from: https://www.fda.gov/media/88496/download [Last accessed on 7 Apr 2023].
33. Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 2015;372:735-46.
34. Phillips RJ, Penington AJ, Bekhor PS, Crock CM. Use of propranolol for treatment of infantile haemangiomas in an outpatient setting. J Paediatr Child Health 2012;48:902-6.
35. European Medicines Agency. Paediatric-use marketing authorisations. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/paediatric-medicines/paediatric-use-marketing-authorisations [Last accessed on 7 Apr 2023].
36. Hachem M, Bonamonte D, Diociaiuti A, Mantuano M, Teruzzi C. Cost-utility analysis of propranolol versus corticosteroids in the treatment of proliferating infantile hemangioma in Italy. PharmacoEcon Ital Res Artic 2015:17.
37. Jadeja N. The role of IP in strategies for repurposing medicines. Available from: http://www.pinsentmasons.com/out-law/analysis/the-role-of-ip-strategies-repurposing-medicines [Last accessed on 7 Apr 2023].
38. Veldman A, Richter E, Hacker C, Fischer D. The use of off-label medications in newborn infants despite an approved alternative being available-results of a national survey. Pharmacy (Basel) 2022;10:19.
39. Ahmed F, Fattani MT, Ali SR, Enam RN. Strengthening the bridge between academic and the industry through the academia-industry collaboration plan design model. Front Psychol 2022;13:875940.
40. European Commission. Commission Expert Group on safe and timely access to medicines for patients (“STAMP”). Available from: https://health.ec.europa.eu/medicinal-products/pharmaceutical-committee-veterinary-pharmaceutical-committee-and-expert-groups/commission-expert-group-safe-and-timely-access-medicines-patients-stamp_en [Last accessed on 7 Apr 2023].
41. European Medicines Agency. Proposal for a framework to support not-for-profit organisations and academia (institutions and individuals) in drug repurposing. Available from: https://www.ema.europa.eu/en/documents/other/question-answers-repurposing-pilot-project-proposal-framework-support-not-profit-organisations_en.pdf [Last accessed on 7 Apr 2023].
42. European Medicines Agency. Building a European innovation platform for the repurposing of medicinal products. Available from: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/horizon-hlth-2021-disease-04-02 [Last accessed on 7 Apr 2023].
43. Hechtelt Jonker A, Hivert V, Gabaldo M, et al. Boosting delivery of rare disease therapies: the IRDiRC Orphan Drug Development Guidebook. Nat Rev Drug Discov 2020;19:495-6.
44. Deplanque D, Fetro C, Ferry A, et al. Drug repurposing: from the discovery of a useful pharmacological effect to making the treatment available to the patient. Therapie 2023;78:10-8.
45. Innovative health initiative. Available from: https://www.ihi.europa.eu/about-ihi [Last accessed on 7 Apr 2023].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Fetro C. Connecting academia and industry for innovative drug repurposing in rare diseases: it is worth a try. Rare Dis Orphan Drugs J 2023;2:7. http://dx.doi.org/10.20517/rdodj.2023.06
AMA Style
Fetro C. Connecting academia and industry for innovative drug repurposing in rare diseases: it is worth a try. Rare Disease and Orphan Drugs Journal. 2023; 2(2): 7. http://dx.doi.org/10.20517/rdodj.2023.06
Chicago/Turabian Style
Fetro, Christine. 2023. "Connecting academia and industry for innovative drug repurposing in rare diseases: it is worth a try" Rare Disease and Orphan Drugs Journal. 2, no.2: 7. http://dx.doi.org/10.20517/rdodj.2023.06
ACS Style
Fetro, C. Connecting academia and industry for innovative drug repurposing in rare diseases: it is worth a try. Rare. Dis. Orphan. Drugs. J. 2023, 2, 7. http://dx.doi.org/10.20517/rdodj.2023.06
About This Article
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.