Sustainable approaches for drug repurposing in rare diseases: recommendations from the IRDiRC Task Force
Abstract
Drug repurposing represents a real opportunity to address unmet needs and improve the lives of rare disease patients. It is often presented as a faster, safer and cheaper path for bringing drugs into new indications. However, several economic, regulatory and scientific barriers can impede the successful repurposing of drugs for rare diseases. The International Rare Diseases Research Consortium (IRDiRC) set up the Task Force on Sustainable Models in Drug Repurposing with the objective of identifying key factors for achieving sustainable repurposing approaches in rare diseases.
In order to help inform expert opinion, the Task Force investigated six cases of medicinal products repurposed into new rare indications and four cases of ongoing development programs. A questionnaire addressing the major steps of the repurposing approach was developed by the Task Force and sent to contact points of the organizations. In addition, interviews were conducted with the relevant organization representatives to conduct a deeper dive into the sustainability of the repurposing approach for each of the selected cases.
Based on the collective experience of the members of the Task Force and the output from the questionnaires/interviews, we have identified ten key factors that should be considered by those embarking on repurposing projects. These factors include the identification of unmet patient needs and partnership with patients, collection of evidence concerning disease prevalence, patient numbers, drug pharmacology and disease etiology, drug industrial property status, off-label or compounding use, data from past clinical studies and needs for extended non-clinical and clinical studies. The development of a collaborative funding framework and early discussion with regulators and payers are additional factors to implement early in the development of sustainable drug repurposing projects.
Keywords
INTRODUCTION
Drug repurposing is a hot topic in regulatory science and drug development. More than ever, the COVID-19 pandemic has clearly shown the importance and potential pitfalls of repurposing existing molecules in the therapeutic armamentarium[1,2].
In the context of rare diseases, a field where the majority of conditions have pediatric onset and the number of therapies is limited[3,4], drug repurposing represents an opportunity for new therapeutic alternatives by exploiting yet untapped pharmacological resources. While previous work done by the IRDiRC Data Mining and Repurposing Task Force had exemplified that the rare disease field could benefit hugely from the repurposing opportunities, the potential is not yet fully realized for a variety of reasons[5].
Repurposing is underused not because of the lack of opportunities, but because the additional resources required to demonstrate the benefit-risk profile of a new indication and sometimes to adapt formulation, posology, and mode of use, often do not translate into a sustainable return on investment via the traditional pharmaceutical industry and healthcare reimbursement models. Indeed, these models are essentially built around innovative, industrial property-protected molecules[6-8], which is defined as one of the two parts of the intellectual property field and relates to the protection of inventions and industrial or commercial creative work that includes patents for inventions, industrial designs, trademarks.
IRDiRC Task Force used the definition of drug repurposing established by the European expert group Safe and Timely Access to Medicinal Products (STAMP)[9]: “Process of identifying a new use for an existing drug/active substance in an indication outside the scope of the original indication”. The Task Force looked at the issue of the under-utilization of repurposing with the specific aim of considering which types of development models could help to foster more successful cases of repurposing while keeping a balance between attractiveness for developers and access to therapies for rare disease patients. The scope included understanding key features of success criteria for the selected cases of repurposing projects and the subsequent reflections of the Task Force on how to optimize these features.
APPROACH AND METHODS
Task Force members were selected based on their multi-stakeholder experience and knowledge of different elements of repurposing programs. The Task Force conducted literature-based reviews to inform the conclusions of this perspective paper. In order to help further inform expert opinions from the Task Force, we selected different types of repurposed cases that would help us further identify key success factors for sustainable repurposing models in rare diseases. Using the collective knowledge of the Task Force, we created a questionnaire that could be used to capture the major steps of each of the repurposing approaches.
Creation of the questionnaire
A targeted questionnaire addressing the following [Supplementary File 1]:
● Description of the company/organization
● Description of the drug repurposing approach and strategy
● Identification of the stakeholders
● Required evidence generation and repurposing of research studies
● Sources and mechanisms of funding
● Sustainability of the model chosen
● Patent status and exclusivity periods
● Identification of the barriers and challenges
● Measure of progress
● Result of the drug repurposing process
● Recommendations for a successful drug repurposing process
Selection of medicinal products
The Task Force extracted a list of repurposed medicinal products that have been approved for a rare indication in the United States[10,11] and/or the European Union[12]. Both oncology and non-oncology drugs were included in the analysis. The Task Force group used a set of five criteria to refine the case selection and create a list of ten repurposed drugs.
● For each selected case, the approval for the original and the repurposed indications must have been granted by the same regulatory body, which is either the United States Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
● Cases related to a variation of the same indication (age, line of treatment) were excluded from the analysis.
● Cases were chosen to avoid the bias of selecting drugs approved for a second indication without the initiation of repurposing studies. This criterion was applied to maximize the probability of identifying cases no longer covered by the initial patent families and/or regulatory exclusivity.
● Diverse economic models (private funds, public funds, social bonds, and venture philanthropy) and drug developers (big pharma, SMEs, academics, patient-led groups, and public-private collaborations) were considered.
● Cases were selected based on the ability of the Task Force members to identify and contact a representative from each organization involved in the repurposing of the medicinal products.
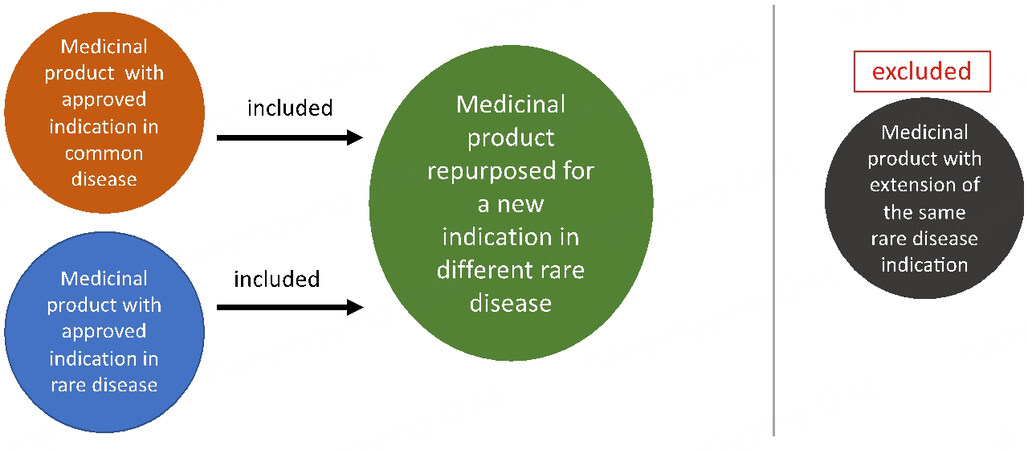
Out of the ten selected repurposed drugs, targeted invitations were sent to six identified organization representatives and six positive responses were received. The questionnaire was sent to the respondents and interviews were conducted with the relevant representatives. The six drugs had initial indications for the treatment of common or rare disorders and were all subject of a repurposed new indication for rare diseases. The selection of medicinal products is presented in Figure 1.
Figure 1. Selection of the Medicinal Products. The graphics represents the methods used by the Task Force group to select cases of drug repurposing in rare diseases. The medicinal products initially approved for common or rare indications were all repurposed into a new rare disease. Approvals for the original and new indication were granted by the same regulatory authority. Medicinal products with an extension of the same rare disease indication were excluded from the analysis.
Case identification of ongoing development programs
Through personal knowledge of the field, the Task Force identified cases of ongoing development programs and contacted four organizations experienced in the management of drug repurposing projects (see acknowledgments section) to perform structured interviews and collect additional information on both the challenges and the key strategies implemented to increase the chance of success of a repurposing approach in rare diseases.
Information from the questionnaires and interviews was integrated into Task Force discussions and the key factors that should be considered by those embarking on repurposing projects were identified and agreed upon through consensus.
KEY FACTORS FOR CREATING A SUSTAINABLE REPURPOSING PROJECT
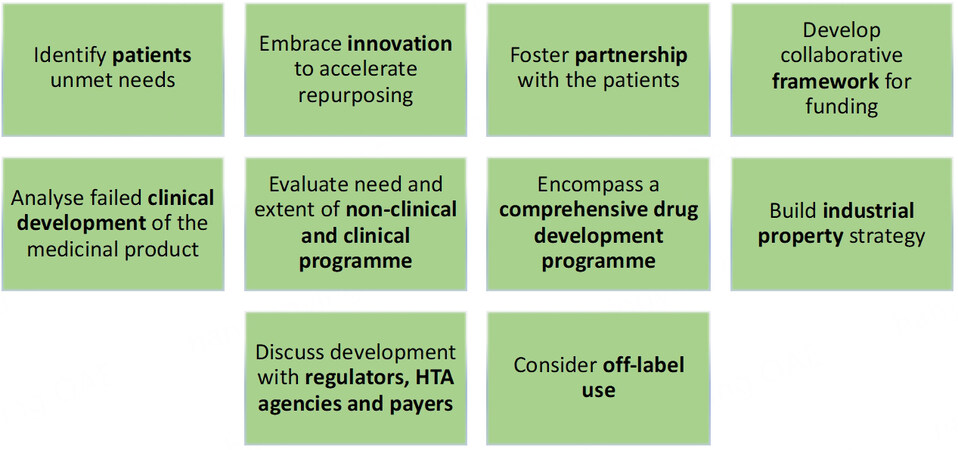
Alongside expert Task Force opinion, the objective of the questionnaire and interview analysis was to draw out common themes not yet considered, and to understand better the challenges and opportunities associated with different models for drug repurposing. The Task Force Group identified ten core success factors that are presented below and summarized in Figure 2.
Figure 2. Ten Success Factors of Sustainable Drug Repurposing Models. The graphic represents ten key success factors to be considered by drug developers embarking on a drug repurposing project. These factors are not presented in a sequential order and form a framework of actions. The identification of unmet patient needs and patient partnerships are key components of sustainability. The collection of evidence concerning disease prevalence, patient numbers, drug pharmacology and disease etiology, drug industrial property status, off-label or compounding use, data from past clinical studies or real world evidence and the need for extended non-clinical and clinical studies is essential in designing a sustainable repurposing project and an accurate business plan. The development of a collaborative funding framework and early discussion with regulators, HTA agencies and payers are also essential factors to implement early on in the development of a sustainable project.
Repurposing is a valid approach to address the unmet needs of rare disease patients
All the respondents, without exception, mentioned that their approach was primarily serving to address the unmet medical needs of the patients, confirming the necessity of a focused and pressing need as a trigger for re-engaging in the development of old drugs. The fulfillment of the patient needs is, in many cases, not only related to the medical and/or scientific aspects of the benefit brought by the product, but also related to the increased availability of the product for the patients.
Alongside the development of therapies for new indications, it was mentioned, for instance, that, for some, the repurposing approach aims at an improvement in the availability of a drug product compared to compounded formulations, alleviating some of the risks linked to the absence of market authorization [with good manufacturing practice (GMP), pharmacovigilance], or the lack of a prescription framework.
Addressing the unmet needs of patients is seen as one of the key components of sustainability. This finding is aligned with foresight scenarios, where repurposing is often considered as an approach linked to social justice as it bears the potential for less costly products that may be accessible to a larger population than the one initially foreseen.
Repurposing is an innovative approach
At a time when the development of gene and cell therapies - and accessibility to such high-end
Some of the approaches that this group, and other stakeholders, patient groups, for example, had classified as repurposing were not labeled as such by pharmaceutical representatives. There is a thin border between what some may call repurposing and the other one, extension of indication. This cannot be imputed only to commercial sensitivities but also to a genuine lack of common understanding and terminology that we should aim to overcome. One understanding of repurposing for companies involved in the development of innovative medicines is that repurposing is considered only for medicinal products that are out of industrial property (IP) and regulatory protection, while the extension of indication is a special type of variation of the initial marketing authorization when new data are submitted and assessed to add a new indication to the existing product.
In addition, repurposing in some quarters suffers from a “bad reputation” where well-known products have seen their prices skyrocketing following a repurposing project[13,14]. This negative perception is not connected to a unique category of stakeholders.
Furthermore, repurposing also brings a positive perception as it is sometimes the only way to provide a therapeutic alternative to patients. Moreover, repurposing, in many cases, is an innovation in itself, where a better understanding of the pharmacology of a medicinal product and the disease etiology reach a point of convergence to stimulate a repurposing project[8,15]. This is increasingly the case where artificial intelligence, data mining and in silico approaches are being used[5]. In order to advance the field, we need to encourage investment and continuous development in this field but also recognize that repurposing is a truly innovative approach.
As for every therapeutic development, patients should be considered as equal partners
Within the sample of interviews, we observed a large variety of patient involvement, from patients and patient organizations being the initiators and the drivers of the repurposing approach to patients only considered at the time of recruitment for the clinical studies.
Amongst the various activities in which patients can take part, we have enlisted: identification of the opportunity to repurpose a product, co-writing of the grant application, provision of the patient perspective in the set-up of the objectives of the therapeutic development, in the design and the planning of the clinical studies, patient identification, recruitment and retention for clinical studies, regulatory preparedness
Often it appeared that the more the patients are involved as equal partners, the better, and that the positive impact is correlated to the level of patient engagement. Interviewees that have reported no or few patients involved in their development mention the identification of stakeholders as one of the main challenges in the repurposing approach, leading to difficulties at the time of recruitment and consequences on the business plan.
Respondents that have reported a high level of patient involvement, with all stakeholders (patients, clinicians, and researchers) inputting in the design and planning of the studies, as well as regulatory preparedness, mention a significant impact on the timeline of the development with faster clinical trial recruitment and better uptake of the product in the market. This is comparable to previous studies that have also emphasized the importance of patient involvement in the development and lifecycle of orphan medicinal products[16-18].
Added value of a collaborative approach in drug repurposing
From the interviews that we have conducted, there are three main patterns that emerge when it comes to funding a repurposing approach.
Firstly, when the project is initiated by a pharmaceutical company, the funding is primarily ensured by the company’s own funding, with the addition of private funding such as capital risks investors on occasion.
Secondly, when the project is initiated by an academic team (researchers and/or clinicians), the project is mostly fuelled by public funding (e.g., European Union/National funding, E-Rare calls, United States federal grants, NIH grants). In that case, it can be complemented by funding from patient organizations and other not-for-profit entities, such as foundations. Additional support can be sought from pharmaceutical companies to supply the drug in clinical trials at no cost, for instance.
The third avenue which breaks the silos existing in the two previously described situations, and that the authors would encourage, is a public-private partnership, as we know that often the discovery phase is done by the public sector while the development phase is performed by the private sector. To make such a public-private approach successful, the expectations of each party and the respective contributions have to be clarified at the beginning of the collaboration, including the signature of contracts and legal agreements.
To make the link with the above item (“As for every therapeutic development, patients should be considered as equal partners”), the involvement and, to a larger extent, a true patient-led approach seem to be critical to ensure a multi-stakeholder repurposing program. The development of a framework for funding and risk in collaborative projects when there are non-commercial opportunities (patent expired) and where private partners would put the responsibility to patients above profit-motive could address some of the needs for sustainable approaches in drug repurposing. As an example, the two Consortia REPO4EU and REMEDI4ALL[19,20] selected following the call by the European Commission under the Horizon Europe program[21] might bring a piece of the answer here.
Learning lessons from failures and building on past findings
The trigger for a repurposing approach can be anything from scientific discovery, data mining or artificial intelligence (AI), clinical observation, off-label use or extensive literature evidence.
It was reported almost unanimously that the collection of scientific evidence in order to support a repurposing approach is a major obstacle. Early detection of the potential for repurposing can represent an advantage and sometimes a competitive one. However, gathering knowledge from past non-conclusive clinical studies in order to inform the new development is not always that easy (e.g., lack of responsiveness of previous investigators, failed clinical trials not published). When it has happened well, it has been highly beneficial.
According to several of the interviewees, a lot of emphases have to be put on the publication of failed clinical trials, the importance of collaboration between the research teams (e.g., the possibility of contacting the initial investigators), and the importance of data sharing which is often identified as one of the main challenges (e.g., non-clinical development information for the product that might support dosing strategies for the repurposed indication).
Generation of additional knowledge is often needed and should not be underestimated
There is a common misbelief that repurposing does not require the extensive generation of new knowledge because there is a huge amount of data that can be re-used from past investigations, clinical studies, and post-authorization generated data.
Not repeating the issue exposed in “Learning lessons from failures and building on past findings”, where the information might exist but the investigator has no access to it, we shall consider two distinct situations: on-target (where the pathophysiological mechanism is known) and off-target (where the mechanism is being discovered) repurposing approaches. The latter offers fewer opportunities to re-use previously generated findings.
Without distinguishing one or the other situations, the interviewees have reported that they had to perform one or more of the following steps: toxicology testing (especially if the new indication is for the pediatric population while the initial one was in adults); non-clinical pharmacology testing (i.e., use of animal models); in vivo non-clinical proof of concept; development of new clinical endpoints and outcome measures, e.g., Patient Relevant Outcomes Measures, Patient Reported Outcomes, and Health-Related Quality of Life Studies.
The conduct of non-clinical research studies and clinical trials is often cited as one of the main challenges. In any case, the generation of additional knowledge is a step that should be factored into the development plan and should not be underestimated.
Repurposing is not always an easy journey, it is a true therapeutic development
To follow on from the previous item, the respondents said that being able to conduct an abbreviated program in the case of a repurposing approach is not granted. In many cases, it was not possible. It is not exceptional to have to do a Phase 1 trial and even develop an animal model.
The development of a new formulation is also often cited. A new formulation is required when reaching a new population (e.g., pediatric versus adult or targeting a specific organ), when a different dosage is needed or when a novel route of administration is explored.
On top of that, repurposing approaches encompass the need for GMP supply and pharmacovigilance follow-up (compared to compounded formulations, for example), which makes it a complete cycle and significantly increases the costs and complexity of the development estimated at first glance.
Hence a repurposing approach should not be underestimated. It is not necessarily always going to be faster than any other type of therapeutic development, and while the length can be reduced due to a certain number of factors (e.g., patient involvement, rigorous planning, extrapolation of data), this is not a given. This finding has been reported by several interviewees.
Defining industrial property strategies require strong expertise and knowledge
IP strategies must be established at the time of the discovery-based first on the patentability of the repurposed drug in its new application and then eventually on the “freedom-to-operate” analysis. The patentability analysis aims at looking into state of the art, meaning any published documents related to the invention including active, abandoned and expired patents besides the scientific literature. Once the patentability of the discovery is established, the path to developing the repurposed drug remains a fine line. Unlike the on-target approach where the pathophysiological mechanism is known, the off-target approach allows the possibility of filing a patent in a second medical use, which can lead to the building of a patent portfolio of higher commercial value for investors. Indeed, interviewees have reported that once the patent of a second medical use is granted, the protection can be further extended via other types of patents: new formulations (i.e., pediatric formulation, medical device, and prodrug), dosage, administration route, and combination of drugs.
Additionally, the non-patentability of the discovery will likely discourage investors. IP protection is thus considered as a factor of success; the objective is to exploit it, and make good use of the advantages it provides, e.g., support the return on the investment.
Finally, IP strategy is a complex field, generating a high level of competition. Patents have to be carefully designed as they are often attacked and dismissed by competitors. For non-specialists, and especially in the case of a repurposing approach conducted by academics, the importance of Tech Transfer Offices is crucial to help navigate the field, avoid pitfalls (e.g., no publication when a patent is sought) and build a strong IP strategy.
Development/Business planning requires defining upfront the end of the journey and each step of the development path
The granting of an orphan designation does not seem to be necessarily a key element in a repurposing approach, whereas the regulatory approval, i.e., granting of a Marketing Authorization, is more often seen as a goal.
Regulatory issues have been reported, such as the need for placebo data which was not anticipated or the importance of choosing endpoints that are relevant for the patients. In those cases, the regulatory system offers tools to overcome such barriers, such as the FDA meeting or EMA Scientific Advice for example. EMA has also opened a pilot on repurposing to support not-for-profit organizations and academia[9]. FDA allows 505(b)(2) application for a not previously approved indication for a marketed product[22].
Market access is seen as a big risk for various reasons. While the availability of the product for patients and the granting of reimbursement are seen as success factors, difficulties are reported in relation to several aspects: (a) the estimate of the target population for which accurate prevalence figures are needed; (b) the anticipation of the generics & compounded products competition and the underestimation of the off-label use which leads to incorrect predictions; and (c) the commercialization costs that might be higher than expected in some countries and might represent an issue if not adequately anticipated.
All this has consequences on the selection and implementation of the economic model, which is seen as one of the main challenges in the repurposing approaches. As a mitigation measure, some interviewees mentioned the need to refine and adapt their business plan during the development path in order to better reflect the R&D costs and the commercialization costs and hence to adjust the price.
The principles expressed in the IRDiRC Orphan Drug Development Guidebook (ODDG) published in 2020[23] are applicable here, i.e., early planning of the development path, best use of all the tools available, early and continued dialogue with regulators and with Health Technology Assessment (HTA)/payers to anticipate the access phase since the beginning. However, the ODDG had not considered the aspects related specifically to repurposing and we aim to add a specific module to the existing materials. This represents a key IRDiRC activity in 2023[24].
Off-label considerations
As mentioned in the previous section, most of the respondents considered the granting of a Marketing Authorization as one of the main aims of their repurposing approach. However, this is not the case for all developers. For some, the success will be determined by the use of the repurposed drug clinically, recommended use and inclusion in well-accepted consensus treatment guidelines, and its acceptance for payment by payers. Further development could then be conducted via an observational study to track its use in the real world instead of an open-label clinical trial.
Considering that generic drugs targeting key pathways are likely to be efficacious in treating a variety of diseases, it was reported that with the appropriate pairing of drugs and untreated or undertreated diseases, drug manufacturers, health care systems, and patients stand to greatly benefit from the identification of repurposing of these medications. This model may not only provide a faster, simpler pathway to providing new therapies for underserved populations, but avoids the cost and time of developing and producing novel therapies.
This point should be balanced by the fact that off-label use is considered very differently depending on the country/region of the world, and such use lacks the advantages of regulatory review on the benefits and risks with formal pharmacovigilance oversight. In some places, it is not authorized by law; in some others, there is widespread access to the (generic) drug off-label at a relatively low cost (of note: when the drug is off-patent, it is more likely that the price will be kept low); in some others, off-label is tolerated but sometimes not reimbursed[25]. In addition, it might also bring fewer opportunities to monitor safety issues and more uncertainty for patients who are then at risk of a withdrawal from the market of the drug for reasons related to the licensed indication, leaving them with no alternative.
DISCUSSION
The over 6,000 rare diseases affect about 300-350 million people worldwide. Low disease prevalence, heterogeneous patient populations, the limited natural history of the diseases and high research and development costs are among the factors limiting the emergence of new therapeutic options for rare disease patients. Altogether, less than 6% of rare diseases have approved treatments, and therefore, drug repurposing must be supported and valued as an approach to address the unmet needs of rare disease patients. This paper aimed to provide drug developers with a framework for creating a sustainable approach to drug repurposing, helping researchers avoid recurrent pitfalls and increasing the chance of seeing medicines repurposed into new orphan indications. Through the Task Force, we have derived ten key success factors which can be considered and applied consistently by developers using repurposing approaches. Based on these ten items, the Task Force group has drawn up three main cross-cutting actionable recommendations: awareness raising, funding allocation, and operational implementation.
What is needed for sustainable approaches in drug repurposing in terms of awareness raising?
Some efforts are required in order to disentangle concerns arising around repurposing. First, there is a need for clarification of what is intended by the term “repurposing” itself. In this respect, the recent manuscript from Asker-Hagelberg et al. brings a welcome precision to the definition of repurposing: “Medicine repurposing is the process of identifying and substantiating a new use for an existing medicine/active substance outside the scope of the original indications as well as the process of allowing a medicinal product to broaden its position in a relevant market (excluding the extension of an authorised indication to those of a new age group or to another genetic mutation)”. The latter part of the sentence clarifies which extensions of indications are to be considered as repurposing and which ones are not[25].
It also clarifies the scope of repurposing: “It includes new therapeutic uses for existing medicines, different formulations of the same medicine, and/or creating new combinations of medicines or medicines with medical devices. Repurposing of medicines is part of the routine research portfolio of both the pharmaceutical industry and academic institutions in the search for solutions for those conditions with unmet medical needs including aspects related to sustainability and patient access”. This is very much aligned with the findings from this Task Force, although few considerations have been given so far to a combination of medicines or medicines with medical devices and should be included in further steps.
The second aspect that should be disentangled is the shadow put on repurposing based on pricing considerations. The authors of this paper believe that this discussion is not specific to repurposing and relates to considerations around value-based assessment versus willingness to pay, and as such, this should not hamper the opportunities offered by repurposing.
Thirdly, it came very strongly from the interviews that neither the benefits brought by repurposing nor the pitfalls that come with it should be underestimated. Despite some aspects of the therapeutic development path being easier than a development starting from scratch, careful consideration should be given to all items identified in the START checklist of the Orphan Drug Development Guidebook[23]: (a) mapping of the stakeholders; (b) analysis of the available knowledge already generated on the disease, the product and the targeted patient population; (c) resources planning in order to anticipate the gaps, the additional data to be generated, the regulatory and market access requirements; and (d) the target patient value profile to always keep the unmet needs of patients in focus.
What is needed for sustainable approaches in drug repurposing in terms of funding orientation?
Over the years, IRDiRC funding member organizations have often been referring to the Consortium recommendations when setting up their work program and calls for funding. Notably, the Horizon Europe work program from the European Commission has proposed HORIZON-HLTH-2022-DISEASE-06-04-two-stage: Development of new effective therapies for rare diseases & HORIZON-HLTH-2021-DISEASE-04-02: Building a European innovation platform for the repurposing of medicinal products[21] which are fully aligned with the roadmap of the IRDiRC Therapies Scientific Committee[26]. The latter provides provision for two consortia[19,20] that are aiming to contribute to the goal presented in this article.
Funding schemes are of utmost importance to allow for a true collaborative partnership to be formed[27-29]. In turn, public-private partnerships are crucial to help cross the so-called valley of death of therapeutic development, which exists for all types of diseases and therapeutic approaches, and which is maybe even more visible in the case of repurposing approaches in the rare disease space.
Funding is not only needed to help progress individual repurposing approaches; there is also a need for funding of pre-competitive activities in order to build infrastructures, foster common knowledge generation (e.g., validate rare disease clinical trial endpoints) and enable sharing of existing and evolving data and information. One avenue for additional investment that came out from the interviews is the lack of accurate figures of prevalence for all rare diseases and in all geographic regions. These figures are not only crucial in the development space of medicines, but also for diagnosis, care and socio-economic planning.
What is needed for sustainable approaches in drug repurposing in terms of implementation?
Access to data generated by previous researchers and developers has been clearly described as a major difficulty during the interviews. The publication of failed or non-conclusive clinical trials would be utterly helpful in the case of repurposing and should be encouraged, as well as the sharing of information amongst researchers, clinicians and pharmaceutical companies. In the European Union, the modification of the Clinical Trials Regulation and the launch of the Clinical Trial Information System early in 2022 will facilitate access to clinical trial data and ensure more transparency to the public[30].
Another area that would need more reflection is related to off-label use. Discrepancies are so striking around the world that it is difficult to draw a single recommendation[25].
Some roadblocks to repurposing approaches do not require additional funding, but rather a better use of already-available tools, incentives and initiatives, as well as a reflection on how to make the system more efficient. For example, co-creation with patients is still not a reality in each and every therapeutic approach and a fortiori in the examples that we saw in this study, despite lots of tools being already available[31]. In addition, new frameworks have been put in place by regulatory agencies, and the opportunities need to be disseminated widely. Regulatory agencies are engaging with stakeholders in order to facilitate drug repurposing. In 2019, the FDA, in collaboration with United States National Institute of Health (NIH) and the Reagan-Udall Foundation, provided an overview of the challenges of drug repurposing while encouraging further development[32,33]. In October 2021, the European Medicines Agency (EMA), in collaboration with the Heads of Medicines Agencies (HMA), launched a pilot project to support the repurposing of medicines by providing a piece of scientific advice for the selected repurposing candidate projects[34].
Bringing repurposed medicines to rare disease patients offers a strong potential to address many unmet needs. This approach, combined with the development of innovative clinical trial designs, can accelerate the emergence of repurposed drugs targeting multiple rare diseases sharing the same molecular etiology. It would also represent a valuable option for the so-called under-researched rare diseases for which unmet needs must be urgently addressed. IRDiRC is tackling these questions with the Shared Molecular Etiologies Underlying Multiple Rare Diseases Task Force and the Pluto Project[35,36]. Drug repurposing is clearly recognized as an opportunity to accelerate the development of therapies for rare diseases and to address Goal 2 of IRDiRC[37]: “1000 new therapies for rare diseases will be approved, the majority of which will focus on diseases without approved options”. The IRDiRC Task Force on Data Mining and Repurposing[5] initially addressed this topic by investigating the potential of biomedical data mining strategies to repurpose and accelerate the development of drugs for rare disease patients. More recently, IRDiRC launched the Drug Repurposing Guidebook Task Force[24] with the objective of describing the tools, incentives, resources and initiatives available to developers in the field. While the key principles of the Guidebook are applicable to the case of repurposing, no specific repurposing building blocks were created at the time and this will be the purpose of the creation of the “Drug repurposing Guidebook” module. This activity will apply the key principles established for the Orphan Drug Development Guidebook Task Force[23] and support more efficient drug development plans.
In conclusion, drug repurposing represents a real opportunity to address unmet needs and improve outcomes for rare disease patients. This IRDiRC Task Force has identified key factors for achieving sustainable repurposing approaches in rare diseases, helping developers optimize their development programs by determining the challenges and also opportunities associated with a particular drug repurposing model or approach.
DECLARATIONS
AcknowledgmentsThe Task Force would like to thank the individuals and organisations (French Foundation for Rare Diseases; Castleman Disease Collaborative Network; University of Pennsylvania, Center for Cytokine Storm Treatment and Laboratory; Cell Therapies Research & Services Laboratory) who gave their time to fill in the questionnaire and/or attend an interview. We are grateful for these reflections which helped formulate the 10 key factors.
Authors ContributionLed the Task Force: Hivert V
Initiated the desk search analysis to identify cases of repurposed medicinal products into new indication in rare diseases: Zanello G
Initiated the redaction of the manuscript: Hivert V, Zanello G
All the co-authors revised and approved the manuscript, and all the co-authors participated in the final selection of use cases, the development of the questionnaire, the interviews and the result analysis.
Availability of data and materialsThe datasets analyzed as sources for the lists are available from the corresponding author upon request.
Financial support and sponsorshipThe IRDiRC Scientific Secretariat is funded by the European Union through the European Joint Programme on Rare Disease under the European Union’s Horizon 2020 research and innovation programme Grant Agreement N°825575. The Scientific Secretariat is hosted at the French Institute of Health and Medical Research (INSERM) in Paris, France.
Conflicts of interestAll authors declared that there are no conflicts of interest. The findings and recommendations in this article are those of the contributors, who participated based on their individual expertise and are responsible for the contents, and do not necessarily represent the views of the members of the International Rare Diseases Research Consortium (IRDiRC) nor any employers of the contributors.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Bellera CL, Llanos M, Gantner ME, et al. Can drug repurposing strategies be the solution to the COVID-19 crisis? Expert Opin Drug Discov 2021;16:605-12.
3. Giannuzzi V, Conte R, Landi A, et al. Orphan medicinal products in Europe and United States to cover needs of patients with rare diseases: an increased common effort is to be foreseen. Orphanet J Rare Dis 2017;12:64.
4. Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet 2020;28:165-73.
5. Southall NT, Natarajan M, Lau LPL, et al. IRDiRC Data Mining and Repurposing Task Force. The use or generation of biomedical data and existing medicines to discover and establish new treatments for patients with rare diseases - recommendations of the IRDiRC Data Mining and Repurposing Task Force. Orphanet J Rare Dis 2019;14:225.
6. Breckenridge A, Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat Rev Drug Discov 2019;18:1-2.
7. Begley CG, Ashton M, Baell J, et al. Drug repurposing: misconceptions, challenges, and opportunities for academic researchers. Sci Transl Med 2021;13:eabd5524.
8. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019;18:41-58.
9. Member States, National Competent Authorities; EU Innovation Offices. Proposal for a framework to support not-for-profit organisations and academia (institutions and individuals) in drug repurposing. Available from: https://ec.europa.eu/health/system/files/2021-10/pharm773_repurposing_annex_en_0.pdf [Last accessed on 11 Apr 2023].
10. Miller KL, Lanthier M. Investigating the landscape of US orphan product approvals. Orphanet J Rare Dis 2018;13:183.
11. U.S. Food and Drug Administration. Search orphan drug designations and approvals. Available from: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/ [Last accessed on 11 Apr 2023].
12. Orphanet Inserm US14. Lists of medicinal products for rare diseases in Europe. Available from: https://www.myobase.org/doc_num.php?explnum_id=10705 [Last accessed on 11 Apr 2023].
13. Postema PG, Schwartz PJ, Arbelo E, et al. Continued misuse of orphan drug legislation: a life-threatening risk for mexiletine. Eur Heart J 2020;41:614-7.
14. Simoens S, Picavet E, Cassiman D, Dooms M. PHP15 What price do we pay for repurposing medicines for rare diseases? Value in Health 2012;15:A15-6.
15. WHO/EURO. Repurposing of medicines in oncology - the underrated champion of sustainable innovation. Available from: https://apps.who.int/iris/bitstream/handle/10665/342567/WHO-EURO-2021-2807-42565-59178-eng.pdf?sequence=1&isAllowed=y [Last accessed on 11 Apr 2023].
16. Aartsma-Rus A, Vroom E, O’Reilly D. The role of patient involvement when developing therapies. Nucleic Acid Ther 2022;32:118-22.
17. Cavaller-Bellaubi M, Faulkner SD, Teixeira B, et al. Sustaining meaningful patient engagement across the lifecycle of medicines: a roadmap for action. Ther Innov Regul Sci 2021;55:936-53.
18. Hoos A, Anderson J, Boutin M, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Ther Innov Regul Sci 2015;49:929-39.
19. REPO4EU. The Euro-global platform for mechanism-based drug repurposing. Available from: https://repo4.eu/ [Last accessed on 11 Apr 2023].
20. REMEDi4ALL. The European Platform for medicines repurposing. Available from: https://remedi4all.org/ [Last accessed on 11 Apr 2023].
21. European Commission. Horizon Europe work programme 2021-2022. Available from: https://ec.europa.eu/info/funding-tenders/opportunities/docs/2021-2027/horizon/wp-call/2021-2022/wp-4-health_horizon-2021-2022_en.pdf [Last accessed on 11 Apr 2023].
22. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Guidance for industry applications covered by section 505(b)(2). Available from: https://www.fda.gov/media/72419/download [Last accessed on 11 Apr 2023].
23. Hechtelt Jonker A, Hivert V, Gabaldo M, et al. Boosting delivery of rare disease therapies: the IRDiRC Orphan Drug Development Guidebook. Nat Rev Drug Discov 2020;19:495-6.
24. IRDIRC. Drug Repurposing guidebook. Available from: https://irdirc.org/drug-repurposing-guidebook/ [Last accessed on 11 Apr 2023].
25. Asker-Hagelberg C, Boran T, Bouygues C, et al. Repurposing of medicines in the EU: launch of a pilot framework. Front Med (Lausanne) 2021;8:817663.
26. Hivert V, Jonker AH, O’connor D, Ardigo D. IRDiRC: 1000 new rare diseases treatments by 2027, identifying and bringing forward strategic actions. Rare Dis Orphan Drugs J 2022;1:3.
27. National Institute of Allergy and Infectious Diseases (NIAID). NOT-AI-16-052: Notice of NIAID’s participation in PA-16-183 “limited competition: Rare Diseases Clinical Research Network (RDCRN) Project supplements for clinical trials to repurpose drugs in collaboration with e-rare awardees (Admin Supp)”. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-AI-16-052.html [Last accessed on 11 Apr 2023].
28. National Center for Advancing Translational Sciences (NCATS); 0. NOT-TR-19-029: NCATS announces the cures acceleration network review board workshop: repurposing off-patent drugs: research & regulatory challenges. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-TR-19-029.html [Last accessed on 11 Apr 2023].
29. ERA-LEARN. E-Rare 3 call for proposals 2016 (JTC 2016): clinical research for new therapeutic uses of already existing molecules (repurposing) in rare diseases. Available from: https://www.era-learn.eu/network-information/networks/e-rare-3/e-rare-3-call-for-proposals-2016-jtc-2016-clinical-research-for-new-therapeutic-uses-of-already-existing-molecules-repurposing-in-rare-diseases [Last accessed on 11 Apr 2023].
30. European Medicines Agency. Clinical trials regulation. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-regulation [Last accessed on 11 Apr 2023].
31. PARADIGM. Patient engagement toolbox. Available from: https://imi-paradigm.eu/petoolbox/ [Last accessed on 11 Apr 2023].
32. U.S. Food Drug Administration. Repurposing off-patent drugs: research & regulatory challenges. Available from: https://www.fda.gov/drugs/news-events-human-drugs/repurposing-patent-drugs-research-regulatory-challenges-12052019-12062019 [Last accessed on 11 Apr 2023].
33. Reagan-Udall Foundation for the FDA. Repurposing off-patent drugs: research & regulatory challenges. Available from: https://reaganudall.salsalabs.org/repurposingoffpatentdrugsworkshop/index.html [Last accessed on 11 Apr 2023].
34. European Medicines Agency. Repurposing of authorised medicines: pilot to support not-for-profit organisations and academia. Available from: https://www.ema.europa.eu/en/news/repurposing-authorised-medicines-pilot-support-not-profit-organisations-academia [Last accessed on 11 Apr 2023].
35. IRDiRC. Shared molecular etiologies underlying multiple rare diseases. Available from: https://irdirc.org/shared-molecular-etiologies/ [Last accessed on 11 Apr 2023].
36. IRDiRC. Pluto Project. Disregarded rare diseases. Available from: https://irdirc.org/pluto-project-disregarded-rare-diseases/ [Last accessed on 11 Apr 2023].
37. IRDiRC. Vision & goals. Available from: https://irdirc.org/about-us/vision-goals/ [Last accessed on 11 Apr 2023].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zanello G, Ardigò D, Guillot F, Jonker AH, Iliach O, Nabarette H, O’Connor D, Hivert V. Sustainable approaches for drug repurposing in rare diseases: recommendations from the IRDiRC Task Force. Rare Dis Orphan Drugs J 2023;2:9. http://dx.doi.org/10.20517/rdodj.2023.04
AMA Style
Zanello G, Ardigò D, Guillot F, Jonker AH, Iliach O, Nabarette H, O’Connor D, Hivert V. Sustainable approaches for drug repurposing in rare diseases: recommendations from the IRDiRC Task Force. Rare Disease and Orphan Drugs Journal. 2023; 2(2): 9. http://dx.doi.org/10.20517/rdodj.2023.04
Chicago/Turabian Style
Zanello, Galliano, Diego Ardigò, Florence Guillot, Anneliene H. Jonker, Oxana Iliach, Hervé Nabarette, Daniel O’Connor, Virginie Hivert. 2023. "Sustainable approaches for drug repurposing in rare diseases: recommendations from the IRDiRC Task Force" Rare Disease and Orphan Drugs Journal. 2, no.2: 9. http://dx.doi.org/10.20517/rdodj.2023.04
ACS Style
Zanello, G.; Ardigò D.; Guillot F.; Jonker AH.; Iliach O.; Nabarette H.; O’Connor D.; Hivert V. Sustainable approaches for drug repurposing in rare diseases: recommendations from the IRDiRC Task Force. Rare. Dis. Orphan. Drugs. J. 2023, 2, 9. http://dx.doi.org/10.20517/rdodj.2023.04
About This Article
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.